
 Brand signature: Orange for biological risk.
Brand signature: Orange for biological risk. Laboratory gloves for biohazard protection.
Laboratory gloves for biohazard protection. Latex gloves and nitrile/polychloroprene gloves.
Latex gloves and nitrile/polychloroprene gloves. Non-sterile and sterile gloves.
Non-sterile and sterile gloves. Maximum comfort.
Maximum comfort. Compliance with the latest European standards.
Compliance with the latest European standards. Category III PPE gloves (PPE Directive 89/686/EEC).
Category III PPE gloves (PPE Directive 89/686/EEC). Extra length from 260 to 300 mm (meets or exceeds EN 420:2003 + A1:2009).
Extra length from 260 to 300 mm (meets or exceeds EN 420:2003 + A1:2009). Bio-safety: AQL 0.65 (EN 374-2:2014 Level 3).
Bio-safety: AQL 0.65 (EN 374-2:2014 Level 3). Virus resistant (ISO 16604:2004 Procedure B and ASTM F1671-97b).
Virus resistant (ISO 16604:2004 Procedure B and ASTM F1671-97b). Extensively tested for chemical permeation (EN 16523-1:2015 supersedes EN 374-3:2003).
Extensively tested for chemical permeation (EN 16523-1:2015 supersedes EN 374-3:2003). Reduced risk of allergies.
Reduced risk of allergies. Packaging designed to comply with laboratory environments processes.
Packaging designed to comply with laboratory environments processes. Double-walled protection afforded by the twinSHIELD™ technology.
Double-walled protection afforded by the twinSHIELD™ technology.

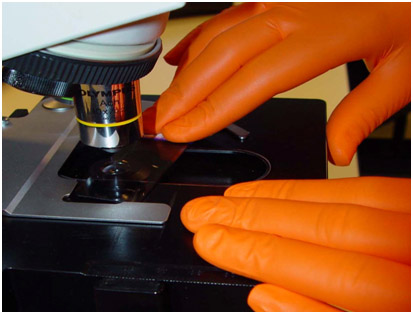
The widely acclaimed SHIELDskin™ gloves provide you with the highest level of “compliance, comfort and protection”. SHIELDskin™ nitrile/polychloroprene gloves have been specifically designed for laboratory use to protect your assays against contamination and to protect you against biological and chemical hazards.
The widely acclaimed SHIELDskin™ gloves provide you with the highest level of “compliance, comfort and protection”. SHIELDskin™ latex and nitrile/polychloroprene gloves have been specifically designed for laboratory use to protect your assays against contamination and to protect you against biological and chemical hazards.
SHIELDskin™ gloves are classified as Category III PPE against the mortal or irreversible risks as defined in the PPE Directive 89/686/EEC. Only Category III PPE gloves are likely to provide the necessary personal protection needed in a laboratory.
SHIELDskin™ nitrile/polychloroprene and latex gloves have been specifically designed for laboratory use to protect your assays against contamination and to protect you against biological and chemical hazards.
A minimum length of 260 mm/10.3″. SHIELDskin™ gloves are the first laboratory gloves to meet the minimum length requirement for liquid proof protection as specified in EN 374-1:2003. By offering 10% extra length compared to standard length laboratory gloves (240 mm) and with extra thickness, they provide optimal protection against biohazards and chemical splashes.
SHIELDskin™ gloves have an Acceptable Quality Level (AQL) of 0.65, representing a performance Level of 3 according to EN 374-2:2014. This level of protection in standard laboratory gloves has never previously been available and will provide additional confidence in meeting biosecurity procedures. Microorganism resistant per EN 374-2:2014 Level 3 (AQL 0.65) and virus resistant per ISO 16604:2004 Procedure B.
SHIELDskin™ ORANGE NITRILE™ gloves are accelerator-free, whilst SHIELDskin™ Bright Latex 300 are free of thiazoles and thiurams thereby limiting the risk of type IV allergic contact dermatitis (or type IV delayed hypersensitivity). Furthermore, there are non-detectable levels of chemical allergens using aqueous solution extraction (Phosphate buffered solution) and High Performance Liquid Chromatography (HPLC) assay method for quantitative analysis.
The SHIELDskin™ ORANGE NITRILE™ gloves are 100% latex-free providing a solution for those seeking to eliminate or minimize the risk of Natural Rubber Latex type I allergy (or type I hypersensitivity).
 Biocompatibility demonstrated by Modified Buehler and Primary Skin Irritation Tests.
Biocompatibility demonstrated by Modified Buehler and Primary Skin Irritation Tests.  Non-detectable levels of chemical allergens using aqueous solution extraction (Phosphate buffered solution) and High Performance Liquid Chromatography (HPLC) assay method for quantitative analysis.
Non-detectable levels of chemical allergens using aqueous solution extraction (Phosphate buffered solution) and High Performance Liquid Chromatography (HPLC) assay method for quantitative analysis.  Free of Thiazoles and Thiurams – these chemical accelerators are excluded from the manufacturing process.
Free of Thiazoles and Thiurams – these chemical accelerators are excluded from the manufacturing process.  Powder free to minimize the potential consequences of powder-borne dermatitis. Residual powder content is 1.0 mg/glove (typical) with a limit of 2.0 mg/glove (ISO 21171:2006 “Medical gloves – Determination of removable surface powder”).
Powder free to minimize the potential consequences of powder-borne dermatitis. Residual powder content is 1.0 mg/glove (typical) with a limit of 2.0 mg/glove (ISO 21171:2006 “Medical gloves – Determination of removable surface powder”).  Micro - organism and virus resistant – passes highest level of micro - organism resistance per EN 374-2:2014 (Performance level 3, AQL
Micro - organism and virus resistant – passes highest level of micro - organism resistance per EN 374-2:2014 (Performance level 3, AQL Tested for electrostatic properties according to EN 1149-1/2/3 & 5.
Tested for electrostatic properties according to EN 1149-1/2/3 & 5. Extensively tested for chemical permeation according to EN 16523-1:2015 (please refer to chemical resistance guide on website - www.shieldscientific.com/public/chemical-resistance-guide).
Extensively tested for chemical permeation according to EN 16523-1:2015 (please refer to chemical resistance guide on website - www.shieldscientific.com/public/chemical-resistance-guide).
| Characteristics | Value | Test Method |
|---|---|---|
| Freedom from holes | < 0.65 AQL1(1 AQL as defined per ISO 2859 for sampling by attributes) | EN374:2003 |
| Tensile Properties | Tensile Strength (min) | Typical Elongation | Ultimate | Test Method |
|---|---|---|---|---|
| Before Aging | 6.0N, min. | 7.0N | 500%, min. | EN455-2:2015 ASTM D573-04(2015) and ASTM D412-15a |
| After Accelerated Aging | 6.0N, min. | 8.0N | 400%, min |
| Dimensions | Measured Point | Mm | MIL | Test Method |
|---|---|---|---|---|
| Nominal Thickness | Middle Finger | 0.17 | 6.6 | ASTM D3767-03(2014) |
| Palm | 0.14 | 5.5 | ||
| Cuff | 0.10 | 4.0 | ||
| Length | 260mm, min. | 265mm, typical | EN420:2003 + A1:2009 |
| Hand Circumference | XS/6 | S/7 | M/8 | L/9 | XL/10 | Test Method |
|---|---|---|---|---|---|---|
| Nominal circumference (mm) | 152 | 178 | 203 | 229 | 254 | EN420:2003+ A1:2009 |
| Palm width | XS/6 | S/7 | M/8 | L/9 | XL/10 | Test Method |
|---|---|---|---|---|---|---|
| Nominal width (mm) | ≤80 | 85 | 95 | 105 | ≥110 | EN 455-2: 2015 |
| Size | Catalogue Numbers | QTY |
|---|---|---|
| Extra Small (XS/6) | 67 6231 | 90 gloves/dispenser, 10 dispensers/case |
| Small (S/7) | 67 6232 | 90 gloves/dispenser, 10 dispensers/case |
| Medium (M/8) | 67 6233 | 90 gloves/dispenser, 10 dispensers/case |
| Large (L/9) | 67 6234 | 90 gloves/dispenser, 10 dispensers/case |
| Extra Large (XL/10) | 67 6235 | 90 gloves/dispenser, 10 dispensers/case |
ShieldSkinTM– Nitrile gloves are manufactured in accordance with ISO 9001:2015 and ISO 13485:2016.
| Material | Proprietary multi-polymer formulation (Acrylonitrile Butadiene with blend of polychloroprene), based on twinSHIELDTM technology.Contains no natural rubber latex. |
| Design | Double barrier protection afforded by orange outer layer, combined with white inner lining. Ambidextrous, beaded cuff and with textured fingertips |
| AQL | AQL 0.65 ( EN 374-2:2014 Level 3) |
| Hypersensitivity | Type I hypersensitivity eliminated - Type IV hypersensitivity reduced. |
| PPE Category | PPE Category III (Complex Design) according to Council Directive 89/686/EEC. |
| Micro - organism Resistance | Passes highest level of micro - organism resistance per EN374-2: 2003 (Performance level 3, AQL <0.65 and inspection level G1 according to 1000ml water test). |
| Viral penetration test | Passes viral penetration test using Phi-X 174 bacteriophage (ISO 16604:2004 Procedure B & ASTM F1671-97b). |
| Chemical permeation test | Extensively tested for chemical permeation according to EN374-3:2003. |
| CE certification | CE 0120 |
Packed with Ninety (90) gloves per dispenser. Dispenser is varnished for enhanced compatibility with laboratory environments. Gloves are flat-packed with ten (10) dispensers per case. Packed in a reinforced single-walled shipping case
SHIELDskin™ is a range of gloves specifically developed for laboratory use and focuses on meeting the needs of users in terms of comfort, compliance and protection. They are registered according to Council Directive 89/686/EEC as Personal Protective Equipment Category III (Complex Design), offering protection to irreversible risk and have been tested according to the 2003 version of the standards. The synthetic gloves in this range are manufactured using a proprietary multi-polymer formulation, whilst the unique unique bicoloured appearance is based on a double dipping process. This twinSHIELD™ technology means that users enjoy double-walled protection. The colour ORANGE, typically associated with higher risk applications, allows for easy colour differentiation.
BioStar offers a complete satisfaction guarantee so you can be confident in your purchasing decision. If for any reason you are not satisfied with the product performance or service provided, we will either replace or issue a refund for the purchase price of your product.
